EFSA Scientific Colloquium 26 on risk-benefit assessment of combined exposure to nutrients and contaminants through food

Risk-benefit assessment (RBA) of food poses significant scientific challenges given the need to weigh the nutritional and health benefits of food types against the potential health risks from hazards such as environmental contaminants present in food. EFSA invites experts in this field to take part in a scientific colloquium on RBA in February 2022.
The discussions and key outcomes of the colloquium will help to inform an update of the EFSA Scientific Committee’s guidance on RBA, published in 2010. The guidance will inform a wide-ranging assessment of the health risks and health benefits of fish consumption, the first of several such planned assessments.
Thank you to all the speakers, experts and participants of our Scientific Colloquium 26 on risk-benefit assessment of combined exposure to nutrients and contaminants through food. All the presentations, documents and videos of the plenary sessions are available below.
Day 1 - 15 February

Presentations
- Maged Younes - Overall Objective of the Scientific Colloquium
- Frans Verstraete - Need for scientific advice on risks and benefits of consumption of food in relation to the presence of contaminants and nutrients
- Morten Poulsen - Current approaches to Risk-Benefit Assessment - Experience gained
- Martin Van den Berg - RBA for the breastfed infant, in relation to the presence of dioxin-like compounds, as determined from the WHO and UNEP global human milk surveys
- Walter Willett - Trends and development in the assessment of nutritional health benefits of consumption of foods
- Wim Verbeke - The influence of trust and perceptions of risks and benefits of consumption of food
- Djien Liem - Introduction to the Break-Out Sessions on 16 and 17 February
Day 2 - 16 February
Day 3 - 17 February
Background
Our scientific knowledge continues to grow about the human health benefits of individual nutrients in foods and the potential adverse health effects of other substances contained in the same foods such as contaminants, residues of pesticides and veterinary drugs. At the same time, the number of questions from risk managers about weighing health benefits and risks of eating these foods is rising.
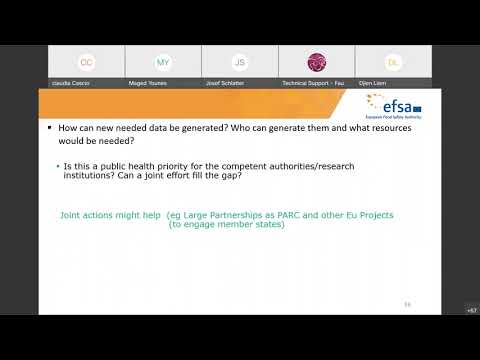
The need for this colloquium was triggered by a request by the European Commission for EFSA to provide an updated RBA of fish consumption in relation to the presence of dioxins and dioxin-like PCBs.
Following consultations with EU Member States and EFSA’s scientific experts, EFSA and the Commission agreed that the assessment should consider a broader group of environmental contaminants: dioxins (PCDD/Fs) and dioxin-like PCBs, methylmercury, brominated flame retardants and perfluoroalkyl substances.
The EFSA Scientific Committee published guidance on health RBA of foods in 2010. Also, FAO/WHO published a report with recommendations for Member States to better assess and manage the risks and benefits of fish consumption and more effectively communicate this to their citizens.
EFSA has decided that an update is needed to incorporate advances in several related scientific fields so that new RBAs can be conducted to provide fit-for-purpose advice to meet the regulatory and risk management needs of the European Commission and Member States in defining dietary advice.
About the colloquium
The scientific colloquium was organised to collect views on current needs and possible approaches for this kind of RBA to serve as input for the Scientific Committee to consider during the update of its guidance document. Specifically, we wished to receive input on advances in RBA methodology, RBA needs, and experience from its application.
The colloquium took place online. The format included plenary and break-out sessions with presentations by internationally renowned keynote speakers. The plenary sessions were streamed live and are available on this page.
Who attended?
Experts in risk-benefit assessment, risk assessors and risk managers from the European Commission and Member States, other national authorities, international organisations including WHO and FAO, as well as interested stakeholders.