Nanotechnology


Nanotechnology is a field of applied sciences and technologies involving the control of matter at the atomic and molecular scale, normally below 100 nanometres. Nanomaterials may exhibit different physical and chemical properties compared with the same substances at normal scale, such as increased chemical reactivity due to greater surface area.
Nanotechnologies enable the management of food ingredients on a molecular level. Nanotechnology products could have a substantial impact on the food and feed sector in the future, potentially offering benefits for industry and the consumer, although possible risks need to be considered. Companies and institutes worldwide are currently researching and developing applications in fields such as the treatment of the mechanical and sensorial properties of food – for instance to achieve changed taste or texture – and modified nutritional value. Nanotechnology may also be used in food packaging, for instance to ensure better protection or to detect how fresh food is. The specific properties and characteristics of nanomaterials need to be considered for any potential health risks.
Latest
EFSA, OECD and JRC, together with the NAMs4NANO project partners developed a graphicto clarify differences between the validation process and the proposed qualification system for New Approach Methodologies (NAMs) developed under the NAMs4NANO project.
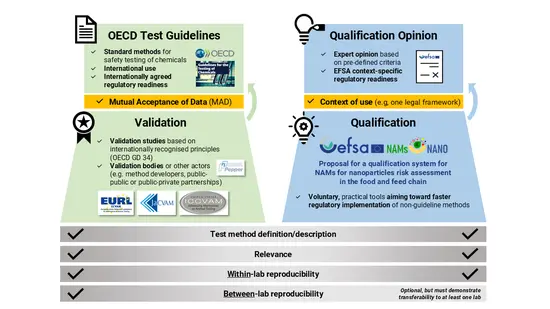
Validation involves the development of standardised test methods (e.g., OECD Test Guidelines) that hold broad regulatory acceptance through the Mutual Acceptance of Data framework.
Qualification offers a more flexible and expert-driven approach based on pre-defined criteria, where scientific validity is assessed for non-guideline NAMs within a clearly defined context of use.
The proposed qualification system would operate on a voluntary basis in areas under EFSA's remit.
Ongoing and completed assessments
Follow EFSA's work
Milestones
2024
EFSA's Scientific Committee begins a new mandate to further update and integrate its nano-related guidance documents. The FEEDAP Panel starts to develop new guidance for use in the risk assessment A specialised field of applied science that involves reviewing scientific data and studies in order to evaluate risks associated with certain hazards. It involves four steps: hazard identification, hazard characterisation, exposure assessment and risk characterisation of feed additives. These developments are scheduled for finalisation over the period 2025-2029.
2023
Nanotechnology was one of four food innovation areas covered at the EFSA ‘Future Food and Feed Lab - Stakeholder workshop on new food/feed sources and technologies’ in March 2023.
2022
November
EFSA discusses implementation of its nano guidance document with feed additive stakeholders.
June
The NAMs4NANO Project takes off aiming to develop methodologies and case studies, and promote the use of New Approach Methodologies (NAMs) in EFSA’s nanotechnology assessments.
April
Stakeholders take part in a nanotechnology workshop focused on information requirements for applicants, and the services and supporting tools available to applicants preparing dossiers for submission to EFSA.
2021
August
Two new EFSA guidance documents – on animal and human health, and on requirements for regulated food and feed product applications further clarify how EFSA’s scientists approach the assessment of nanomaterials in the food and feed chain. They set down data and information requirements for applicants submitting materials for assessment as part of EU market authorisation procedures.
May
EFSA holds a scientific colloquium on “A coordinated approach to assessing the human health risks of microplastics and nanoplastics in food”.
2020
November
EFSA publishes an external report on existing guidance and other published sources related to the environmental risk assessment of nanomaterials. The report will form the basis for EFSA future guidance on assessing environmental risks from the application of nanoscience The study of nanomaterials and nanotechnology in the food and feed chain.
2018
July
New guidance comes out on assessing the safety for humans and animals of nanoscience and nanotechnology applications. It gives practical advice on the testing and methods to apply.
2016
June
EFSA’s scientists provide a state of the science overview on microplastics and nanoplastics as contaminants in food.
2011
May
A guidance document explains how EFSA’s Panels should assess potential risks related to certain food-related uses of nanotechnology.
2010
EFSA’s Advisory Forum establishes the Nano Network to develop cooperation and networking with Member States on nanoscience.
2009
March
EFSA’s Scientific Committee publishes a scientific opinion on nanoscience and nanotechnologies in relation to food and feed safety.
EFSA's role
Since 2006 EFSA has been following developments in nanotechnology within its remit – providing independent scientific advice and technical support to risk managers – including reviewing the current state of knowledge and latest developments in nanotechnology regarding food and feed.
EFSA’s Scientific Committee provides scientific advice on how to assess applications from food operators to use (engineered) nanomaterials in food additives, enzymes, flavourings, food contact materials, novel foods, food supplements, feed additives and pesticides. The work considers the risks of nanomaterials and nano particles that might be present in the food chain, for human and animal health. In the future, this advice will be extended to the assessment of the environmental impact of nano particles.
EFSA’s scientific panels consider the Scientific Committee’s advice in their safety assessments of specific nanomaterials, for instance in the areas of food additives, novel foods and food contact materials. This includes existing guidance documents and new annexes added to address specific technical issues as they arise.
- Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health
- Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticle
- Annex A - Degradation/dissolution rate under acidic conditions
- Annex B - Clarification on the use of a Kow-based threshold A dose or exposure below which adverse effects are not detected for lipid Fat and fat-like substance-soluble (lipophilic) substances as a possible appraisal route
- Annex C - Practical instructions for reporting the results of electron microscopy analysis of nanomaterials or materials possibly containing a fraction of small particles including nanoparticles
- Annex D - Reporting template for an electron microscopy analysis and results
- Use of Dynamic Light Scattering (DLS) and other light scattering methods for characterisation of particle size distribution (see Annex I - 50th meeting of the Working Group on Nanotechnologies)
This advice may also be taken into account by EFSA’s Nano Network, which furthers cooperation and networking between EFSA and Member States on nanoscience and nanotechnology in relation to food and feed safety risk assessment. The network facilitates the exchange of information and expertise, enhances dialogue and builds mutual understanding of risk assessment principles between EFSA and the Member States. We publish an annual report of the network’s activities.
- Applicants and other stakeholders who require support on how to apply the guidance document on nanotechnology, can submit a request via the ‘Ask EFSA a Question’ service.
EU framework
The European Union is taking an “integrated, safe and responsible approach” to the development of nanotechnologies. This includes:
- Creating the European Union Observatory for Nanomaterials (EUON), which among other things monitors safety issues and engages in dialogue with national authorities, stakeholders and citizens.
- Reviewing and adapting EU laws e.g. the European Commission recommendation on the definition of a nanomaterial.
- Nanotechnology – European Commission, DG Health and Consumers
- Research topics: Nanotechnology – Joint Research Centre
FAQ
Nanotechnologies involve, among other things, the use in the food and feed chain of substances of a very small size. A nanometer (nm) is one-billionth of a metre (the term comes from the Greek word nanos, dwarf). Chemicals are generally considered to be nano sized if they are around 100 nm or less in size.
Chemicals that are very small can have different properties compared to the larger sized versions. This can offer opportunities for manufacturing products such as medicines, cosmetics and foods, with the potential to behave differently in useful ways. However this changed behaviour compared to larger sized chemicals could also raise possible risks.
Nanotechnologies have a range of actual or potential uses. For example sunscreens are available that use chemicals that at a nano size make the sunscreen transparent rather than opaque, but still block UV rays. In the food area, it could be possible to use nano-sized chemicals to improve food packaging or enhance the nutritional value of a product.
Because there could be risks from nano sized chemicals due to characteristics and properties that aren’t observed for the larger size versions of chemicals. The use of nano sized chemicals, whilst offering potentially useful applications and benefits, needs also to be considered for any possible risks. That is EFSA’s role in relation to the food chain. EFSA provides independent scientific advice to risk managers in Europe to help them decide on any appropriate action to protect the consumer.
The approval of products sold in Europe is the responsibility of the EC and Member States. EFSA would not be in a position to know about what is on the market as it is not our responsibility, but the technology exists for some applications, and products may be available from outside of Europe that could contain nano sized substances either in the product or its packaging.
Regulation is not within the remit of EFSA, which provides independent scientific advice to risk managers. It is the responsibility of risk managers to consider appropriate measures and assess existing legislation, in light of EFSA’s opinions. Information from the EC on the regulatory aspects of nanomaterials.