Fourth joint inter-agency report on integrated analysis of antimicrobial consumption and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the European Union (JIACRA IV – 2019–2021)
Disclaimer
- This is a simplified summary of ECDC’s, EFSA’s and EMA’s Fourth joint inter-agency report on integrated analysis of antimicrobial agent consumption and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA. The full report can be found here.
- The purpose of this simplified summary is to enhance transparency and inform interested parties on the work of ECDC, EFSA and EMA on the topic using simplified language to present a summary of the main findings.
Background
- Antimicrobial resistance (AMR) is a major global threat to human and animal health.
- The use and misuse of antimicrobials in humans and in food-producing animals are major drivers of AMR. Addressing AMR requires a coordinated effort from the human and animal sectors across the globe.
- Antimicrobial-resistant bacterial infections are a serious health problem in Europe, causing over 35,000 deaths annually. This is comparable to the combined impact of influenza, tuberculosis and HIV/AIDS. Recent data show that antimicrobial-resistant bacteria are causing a growing number of infections and deaths in humans, particularly in healthcare settings.
- In accordance with the European One Health Action Plan against Antimicrobial Resistance, the European Commission (EC) tasked the European Centre for Disease Prevention and Control (ECDC), the European Food Safety Authority (EFSA) and the European Medicines Agency (EMA), to gather data on the link between antimicrobial consumption (AMC) and AMR in humans and food-producing animals.
- This is the fourth Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) report, primarily covering the period 2019–2021. The three previous JIACRA reports considered AMC and AMR data for a series of consecutive periods since 2011.
What were ECDC, EFSA and EMA asked to do?
- The EC tasked ECDC, EFSA and EMA to produce the fourth JIACRA report. The report presents results of data analyses that investigate possible associations between AMC in humans and food-producing animals and the occurrence of AMR in bacteria in both sectors. The analyses also sought to identify significant trends in AMR and AMC and assess whether trends were concomitant.
How did ECDC, EFSA and EMA carry out this work?
- The fourth JIACRA report was produced by considering different sets of data originating from the EU-wide surveillance and monitoring programmes of AMR and AMC in humans and food-producing animals in the European Union (EU) and European Economic area (EEA), respectively coordinated by ECDC, EFSA and EMA. Data from 2019 to 2021 formed the primary basis for the analysis (Figure I).
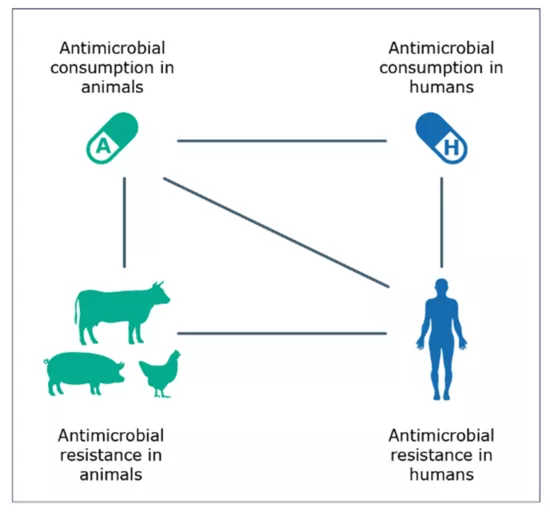
Figure I: Potential links investigated between antimicrobial consumption in humans and food-producing animals and antimicrobial resistance in bacteria from humans and food-producing animals
- To analyse and compare AMC, data on total consumption of antimicrobials from EU/EEA countries were combined to calculate an average consumption in milligrams of antimicrobials consumed per kilogram of estimated biomass of humans and of food-producing animals. Minor technicalities in the different metrics used within the specific surveillance programmes of AMC in humans and in food-producing animals may have affected complete comparability between sectors.
- To examine the possible associations between AMC and AMR, a series of analyses was conducted on specific combinations of bacteria and groups of antimicrobials, using available surveillance data from both human and animal sectors. The combinations selected were prioritised according to their relevance to public health and addressed four bacteria and seven groups of antimicrobials (Figure II). Bacterial isolates from humans were from clinically ill individuals sampled in healthcare settings, whereas isolates from food-producing animals were sampled from domestically produced healthy animals at slaughter.
- The same data sources and metrics were used as in previous JIACRA reports, facilitating a trend analysis performed over the period 2014-2021, to identify significant trends and compare trends in AMC and AMR in each country and assess whether trends were concomitant.
What were the outcomes and their implications?
- In 2021, the total AMC in 29 EU/EEA countries was assessed at 125.0 mg per kg of biomass (28 countries, range 44.3–160.1) for humans and 92.6 mg per kg of biomass for food-producing animals (29 countries, range 2.5–296.5).
- Between 2014 and 2021, the mean total AMC in mg per kg food-producing animals was decreased by 44%, while in humans, it remained relatively stable. AMC also varied markedly among EU/EEA countries with most but not all countries exhibiting a decreasing trend. This shows that measures implemented to reduce AMC in food-producing animals and in humans have been effective in many countries. Nevertheless, these measures need to be reinforced so that the reductions are retained and further continued, where necessary. Further efforts would be needed to reduce unnecessary antimicrobial use in humans. Increasing completeness in the reporting of AMC data from humans over the study period might, however, have masked reductions.
- Between 2014 and 2021, in 10 out of 20 countries that significantly reduced total AMC in food-producing animals, the indicator gut bacterium E. coli from food-producing animals became increasingly susceptible to antimicrobials (i.e., exhibiting ‘complete susceptibility’ or ‘zero resistance’ to a harmonised set of important antimicrobials). Similarly, in nine out of 19 countries that significantly reduced total AMC in humans, the E. coli originating from human invasive infections became increasingly susceptible (i.e., exhibiting ‘complete susceptibility’ or ‘zero resistance’ to a harmonised set of important antimicrobials). These results show that reducing AMC overall can help lower AMR. This also highlights the importance of measures that promote human and animal health, such as vaccination and better hygiene, thereby reducing the need for antimicrobials.
- Data from 2019 to 2021 confirmed a link between the levels of consumption of certain groups of antimicrobials and the occurrence of AMR to these groups of antimicrobials in bacteria from both humans and food-producing animals (Figure II):
- In humans, positive associations were found between the level of AMC of carbapenems, 3rd- and 4th-generation cephalosporins and (fluoro)quinolones and resistance to each of these groups in E. coli originating from human invasive infections.
- In food-producing animals, positive associations were shown between the level of AMC of (fluoro)quinolones, polymyxins, aminopenicillins and tetracyclines and resistance to each of these groups in indicator E. coli from food-producing animals.
- In poultry, the levels of consumption of (fluoro)quinolones were positively associated with those of resistance to this group of antimicrobials in Campylobacter jejuni.
- In pigs, the levels of consumption of (fluoro)quinolones and macrolides were positively associated with those of resistance to the respective groups in Campylobacter coli.
- In some cases, AMR in bacteria from humans was also linked to AMR in bacteria from food-producing animals, which were in turn associated with corresponding AMC in food-producing animals, especially for the combinations involving food-borne zoonotic bacteria such as Campylobacter jejuni and (fluoro)quinolones and for Campylobacter coli and macrolides.

Figure II: Links identified in the analysis between antimicrobial consumption (AMC) in humans and food-producing animals and antimicrobial resistance (AMR) in bacteria from humans and food-producing animals
- In food-producing animals, statistically significant reductions in the consumption of 3rd- and 4th-generation cephalosporins, quinolones, polymyxins, aminopenicillins and tetracyclines were registered during 2014–2021 in at least one quarter of countries analysed. For most of these countries, this was accompanied by a trend in reduction of resistance to the related antimicrobial group in E. coli from animal gut samples, and this trend was often statistically significant.
- Over the same period, there were statistically significant trends showing reduction in human consumption of quinolones and aminopenicillins for at least two-thirds of countries included in the analysis. For at least one quarter of these countries, this was accompanied by a statistically significant trend in reduction of resistance to these antimicrobial groups in E. coli originating from invasive infections in humans.
- A statistically significant increase in consumption of carbapenems in humans (mostly from very low levels) was shown in 11 countries. No statistically significant trends were found for the consumption of 3rd- and 4th-generation cephalosporins in humans.
- These data further underscore the need for the continued integrated monitoring of AMC and AMR coupled with interventions aiming at a more prudent use of all antimicrobials, particularly those antimicrobials which are of critical importance in human medicine.
What are the key recommendations?
- Continued and coordinated action is needed to achieve, by 2030, a 20% reduction of AMC in humans (compared to 2019 levels) and a 50% reduction in food-producing animals (compared to 2018 levels), as recommended by the Council of the European Union and as set in the Farm-to-Fork strategy, respectively, including training of health and veterinary professionals.
- Specific efforts should be undertaken to reduce and limit AMC overall both in human and animal sectors, as decreases in overall AMC have been associated with decreases in overall AMR. Particular attention should be also paid to limiting the use of the most important antimicrobials (as defined by EMA's Antimicrobial Advice Ad Hoc Expert Group) in human medicine.
- Increased focus on preventive measures and infection control is crucial to reduce the need for antimicrobial treatments and prevent the spread of AMR.
- Responsible and prudent use of antimicrobials should be ensured, and efforts should be made to improve the availability, accessibility and compliance to diagnostic testing and treatment guidelines.
- Complementary data should be collected for better analysis of the relationships between AMC and AMR through integrated and harmonised surveillance of AMC and AMR in the human and animal sectors. Data from the environmental sector could also be integrated into the analysis, once available, in a ‘One Health’ approach.
- Further targeted studies are needed to investigate and better understand the transmission of antimicrobial-resistant bacteria and AMR genes between animals and humans, as well as the environment.

Figure III: Overview of key recommendations
Glossary
Antimicrobial resistance – means the ability of micro-organisms to survive or to grow in the presence of a concentration of an antimicrobial agent which is usually sufficient to inhibit or kill micro-organisms of the same species (Regulation (EU) 2019/6 – Art. 4(11))
‘Complete susceptibility’ or ‘zero resistance’ – is technically defined as susceptibility to all antimicrobial groups included in harmonised panels of antimicrobials addressed within the framework of a monitoring (panels are presented in the report).
Related resources
Collection of JIACRA reports in the EFSA Journal
European One Health Action Plan against Antimicrobial Resistance
Council’s Recommendation on stepping up EU actions to combat AMR in a One Health approach
EMA web resources:
European Surveillance of Veterinary Antimicrobial Consumption (ESVAC): 2009 - 2023
Page on analysis of antimicrobial consumption and resistance
ECDC web resources:
Antimicrobial consumption dashboard
Antimicrobial consumption reports
Antimicrobial resistance reports:
https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data
https://www.ecdc.europa.eu/en/campylobacteriosis/antimicrobial-resistance
Reference
European Centre for Disease Prevention and Control (ECDC), European Food Safety Authority (EFSA) and European Medicines Agency (EMA). Fourth joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA. Stockholm, Parma, Amsterdam: ECDC, EFSA, EMA; 2024. DOI: https://doi.org/10.2903/j.efsa.2024.8589
ISSN: 1831-4732
© European Food Safety Authority, 2024
Reproduction is authorised provided the source is acknowledged.